How Many Outer Level Electrons Do Lithium and Potassium Have
Metals can have a valency of 12 or 3 only. See answer 1 Best Answer.

Alkali Metals Properties Periodic Table Ios App Chemistry Periodic Table App
It has a very low melting point.
. Elements with a single electron in their outer energy level such as lithium Li sodium Na and potassium K are highly. Let students know that these electrons in the outermost energy level are called valence electrons. There are 2 electrons on the first energy level 8 electrons on the second level 8 electrons on the third energy level and 1 on the fourth energy level.
How Many Outer Electrons Does It Have. Lithium has 1 and Potassium also has one. The 1s electrons are core electrons.
Does lithium lose or gain valence electrons. 2 What is the outer electronic configuration of iron. We know that a potassium atom has a total of nineteen electrons.
In this step the electrons of potassium have to be arranged. 6 How many outer electrons does iron have. 7 What is the electron configuration for iron atomic.
The first and lowest energy level can hold how many electrons. Let students know that these electrons in the outermost energy level are called valence electrons. They tend to lose the single electrons in their outer level.
It has 19 electrons and 19 protons with one valence electron in the outer shell. 1 How many electrons are in the outermost level. Has a full outer energy level shell.
For instance hydrogen lithium sodium and potassium all have 1 electron on their outer energy level. The electron configuration of lithium shows that there are two electrons in the K shell and one in the L shell. Lithium has atomic number of 3.
How many electrons are in potassium outer shell. Students should realize that each atom in a group has the same number of electrons in its outermost energy level. C-3 Valence Electrons and Molecules.
Lithium has 1 and Potassium also has one. The second energy level can hold up to how many electrons. Students should realize that each atom in a group has the same number of electrons in its outermost energy level.
Lithium an alkali metal with three electrons is also an exception to the octet rule. So a neutral lithium atom has 3 protons and 3 electrons. A full valence shell is the most stable electron configuration.
How many outer-levels electrons do lithium and potassium have. 3 How do you find the outer number of electrons. Element Atomic Number Number of electrons in the outermost energy level Number of empty spaces in the outermost level Lithium Potassium Fluorine Bromine Neon Krypton.
4 How many inner outer and valence electrons are in FE. How many outermost electrons do lithium and potassium have. Step 2 is very important.
There are two ways in which atoms can satisfy the octet rule. Two electrons in shell 1 and one electron in shell 2. For instance hydrogen lithium sodium and potassium all have 1 electron on their outer energy level.
Lithium tends to lose one electron to take on the electron configuration of the nearest noble gas helium leaving it with two valence electrons. For instance hydrogen lithium sodium and potassium all have 1 electron on their outer energy level. What do lithium and potassium have.
All the Group 1 elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr have one electron in the outer shell. So Lithium has only 1 valence electron in the 2s orbital. The Group 7 elements fluorine F chlorine Cl bromine Br iodine I and astatine At.
1 How Many Electrons Are in the Outermost Level. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron configuration. It is the second least dense metal after lithium.
Explain that potassium has 19 protons and 19 electrons. We know that lithium atoms have a total of three electrons. In this step the electrons of lithium have to be arranged.
Let students know that these electrons in the outermost energy level are called valence electrons. Group 18 elements helium neon and argon are shown have a full outer or valence shell. The full electronic configuration of potassium is 1s22s22p63s23p64s1.
An energy level can only hold a certain number of electrons. To find this out justlook at the columns in the Periodic Tablethe tell you the numberof valence electrons. 5 How many electron configuration does iron have.
The electron configuration of potassiumK shows that the first shell of potassiumK has two electrons the second shell has eight electrons the 3rd shell has eight electrons and the 4 th shell has an. Potassium atoms have 19 electrons and 19 protons with one valence electron in the outer shell.

Chemistry291 Hand Note 5 Steps How Many Valence Electrons Does Sodium Electrons Electron Configuration Chemical Equation

Atom Model The Element Lithium Li 3 Made Of Two Metallic Circles And Six Clear Balls Atom Model Atom Model Project Atom Project
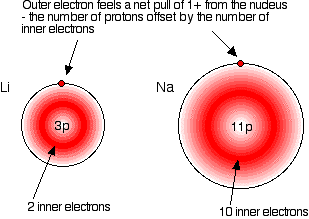
Group 1 Properties Of Alkali Metals Chemistry Libretexts

When Compared To Lithium It Is Easier To Remove Valence Electron From K Potassium Because Youtube

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Potassium Atom Atom Diagram

Periodic Table Worksheet Answers Chemistry Basics Chemistry Lessons Chemistry Worksheets
Chem4kids Com Potassium Orbital And Bonding Info
Solved What Is Common Among Lithium Sodium And Potassium

Reactivity Series Reactivity Of Chemistry Education Chemistry Lessons Chemistry Basics

A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Table Youtube Chemistry Lessons Chemistry Basics Periodic Table

Group 1 Alkali Metals Gcse The Science Hive

Cations Common Metal Cations Names And Formulas Transition Metal Alkali Metal Periodic Table

Acids Bases And Salts All Are Electrolytes Chemistry Lessons Chemistry Education Chemistry Basics
What Will Be The Electron Arrangement For Lithium If The Atomic Number Is 4 Quora

Valency Of Lithium How Many Valence Electrons Does Lithium Li Have
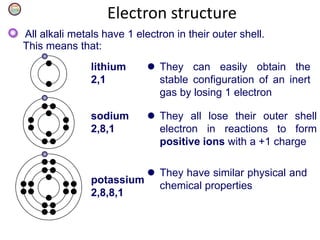
Chemistry Of Alkali Metals Manik
Why Do Lithium Sodium And Potassium Have Similar Chemical Properties Quora

Potassium Has A Nuclear Charge Many Times Greater Than That Of Lithium Why Is It Actually Easier For A Potassium Atom To Lose Its Valence Electron Than It Is For A Lithium
Comments
Post a Comment